Zynomi Clinical Data Platform
Smarter Trials. Faster Outcomes.
Zynomi is a governed, AI-assisted clinical data platform that accelerates trial design, improves data quality, and connects effortlessly to dashboards, BI tools, and regulatory pipelines.
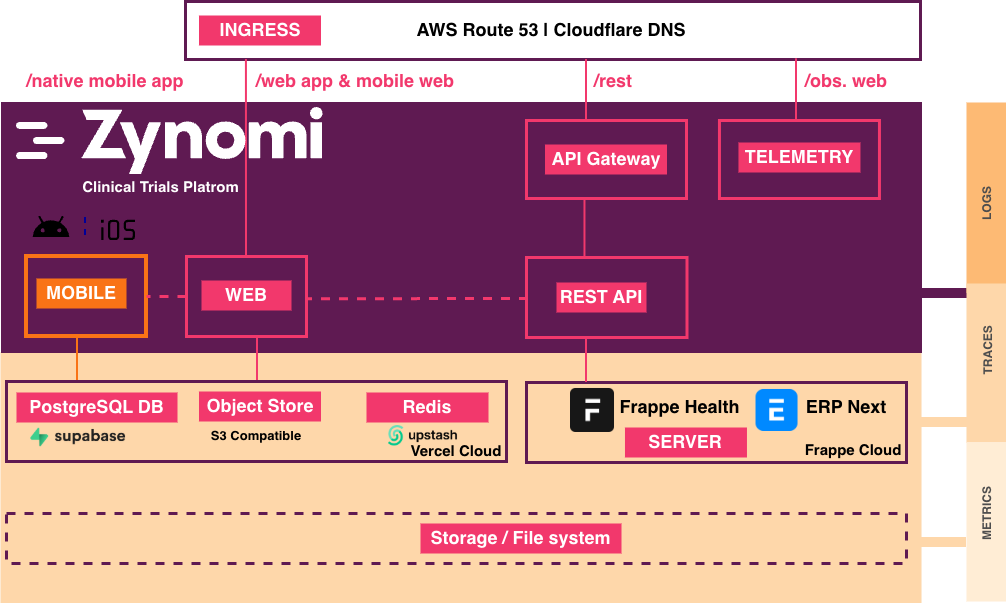
Platform Components
The Zynomi platform brings together applications, APIs, and analytics into a unified clinical trial ecosystem:
-
Zynomi CTMS — Web-based Clinical Trial Management System for study design, site management, eCRF building, and clinical data capture.
-
Sublink — Mobile companion app for subject engagement, medication reminders, ePRO collection, and health diaries.
-
REST APIs — 100+ API endpoints powering integrations with external systems, custom applications, and third-party tools.
-
Semantic Layer — Governed metrics via Cube.dev providing consistent, trusted analytics across all data consumers.
-
MCP Server — Model Context Protocol enabling agentic analytics with AI assistants like Claude, Copilot, and Cursor.
Agentic Analytics
The MCP-powered semantic layer unlocks advanced analytics possibilities including embedded analytics, ad-hoc reporting, natural language queries, multi-modal visualization, and bring-your-own-viz integration with Power BI, Tableau, Superset, or any BI tool of your choice.
Key Capabilities
Study Execution
Zynomi CTMS powers end-to-end clinical trial management:
- eCRF Designer — Metadata-driven form builder for clinical data capture
- Multi-Site Trials — Centralized management across sites, roles, and access levels
- Visit Scheduling — Protocol-driven visit calendars and reminders
- Adverse Event Tracking — Safety monitoring with CDISC-compliant structures
Subject Engagement
Sublink mobile app keeps subjects connected and compliant:
- Medication Reminders — Push notifications for dose adherence
- ePRO Collection — Patient-reported outcomes via mobile app
- Visit Notifications — Appointment reminders and check-ins
- Health Diaries — Daily symptom and activity logging
Analytics and Compliance
Analytics Lakehouse delivers governed insights and regulatory readiness:
- Semantic Layer — Governed metrics via Cube.dev for consistent analytics
- CDISC Domains — Automated generation of DM, AE, CM, VS, LB domains
- BI Integration — Connect Power BI, Tableau, Superset, or Metabase
- AI/MCP Ready — Natural language queries via Model Context Protocol
User Personas
| Role | Description | Application |
|---|---|---|
| Platform Administrator | Full system access, user and master data management | CTMS Web |
| Study Designer | Designs protocols, eCRFs, and configures clinical trials | CTMS Web |
| Principal Investigator | Oversees clinical trials at their site | CTMS Web |
| Study Coordinator | Executes studies, records subject progress | CTMS Web |
| Subject | Logs medications, views reminders, manages profile | Sublink Mobile |
For detailed role responsibilities and permissions, see User Personas and Roles.